5.2 Radioactivity
5.2.2 The three types of nuclear emission
CORE Content
1. Emission of Radiation from a Nucleus
- Radioactive decay is the process by which unstable atomic nuclei lose energy by emitting radiation.
- This process is:
- Spontaneous – It happens without external influence (not affected by temperature, pressure, etc.).
- Random in direction – The emission of particles occurs in unpredictable directions, and it's impossible to know exactly when a particular atom will decay.
- There are three main types of nuclear radiation emitted from the nucleus:
- Alpha (α) particles
- Beta (β) particles
- Gamma (γ) radiation
2. Identifying α, β, and γ Emissions from the Nucleus
(a) Nature of Each Type of Emission
- Alpha (α) particle: A helium nucleus – consists of 2 protons and 2 neutrons.
- Beta (β⁻) particle: A high-speed electron emitted from the nucleus when a neutron changes into a proton and an electron.
- Gamma (γ) radiation: A high-energy electromagnetic wave emitted from the nucleus, often after alpha or beta decay.
(b) Relative Ionising Effects
| Type | Charge | Speed (approx.) | Ionising Power |
|---|---|---|---|
| α | +2 | ~3 × 10⁷ m/s | Very strong |
| β⁻ | –1 | ~2.9 × 10⁸ m/s | Moderate |
| γ | 0 | 3 × 10⁸ m/s | Very weak |
- Ionisation happens when radiation knocks electrons out of atoms, turning them into ions.
- Alpha particles cause dense ionisation in matter due to their large charge and slow speed.
- Beta particles have less ionising power because they are lighter and faster.
- Gamma rays, having no charge and high speed, are the least ionising.
(c) Relative Penetrating Abilities
| Type | Penetrating Ability | Absorbed By |
|---|---|---|
| α | Lowest – travels ~5 cm in air | Paper or human skin |
| β⁻ | Moderate – travels through air & paper | Few mm of aluminium |
| γ | Highest – very deep penetration | Several cm of lead / metres of concrete |
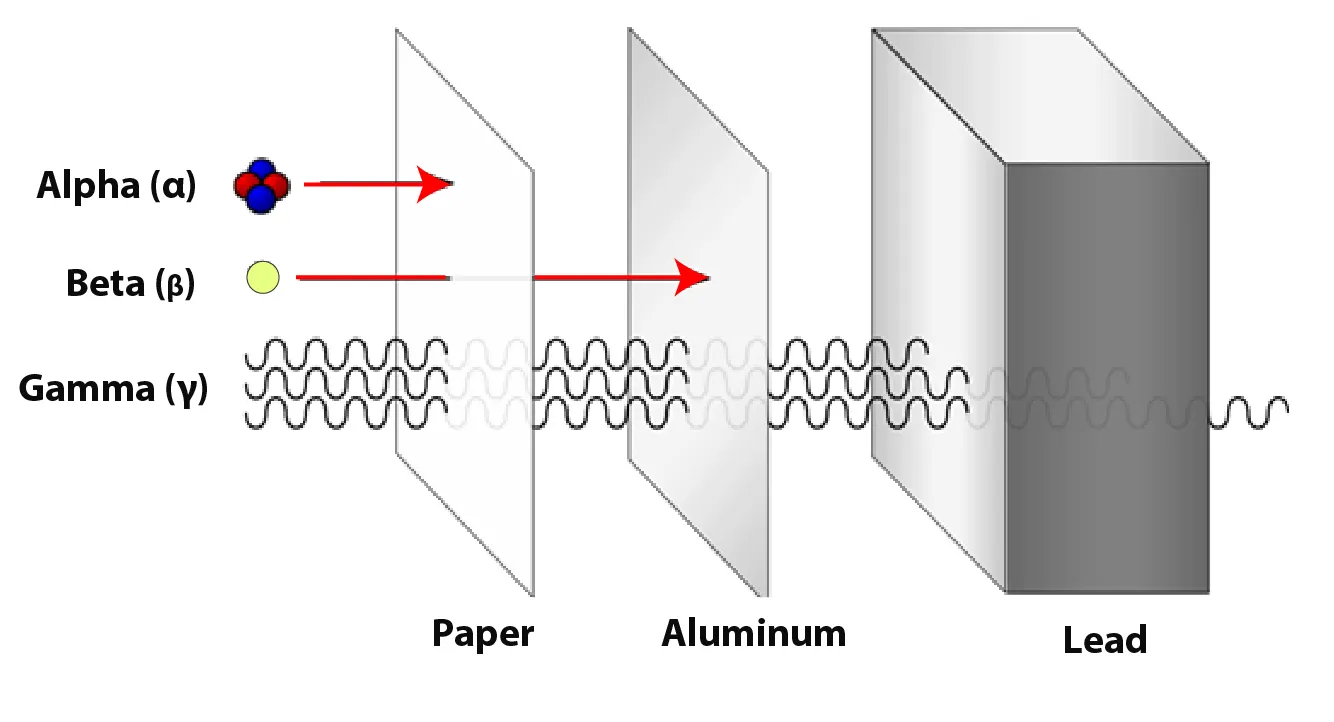
Figure 1: Penetration comparison of α, β and γ
SUPPLEMENT Content
3. Deflection in Electric and Magnetic Fields
a. Electric Field
- Because α and β particles are charged, they are deflected by electric and magnetic fields, but in opposite directions due to their opposite charges.
- α-particles are positively charged (+2), so they are attracted to the negative plate.
- β-particles are negatively charged (–1), so they are attracted to the positive plate.
- γ-rays are uncharged, so they are not deflected by electric or magnetic fields.
- β- particles are deflected more than α-particles as they are lighter.
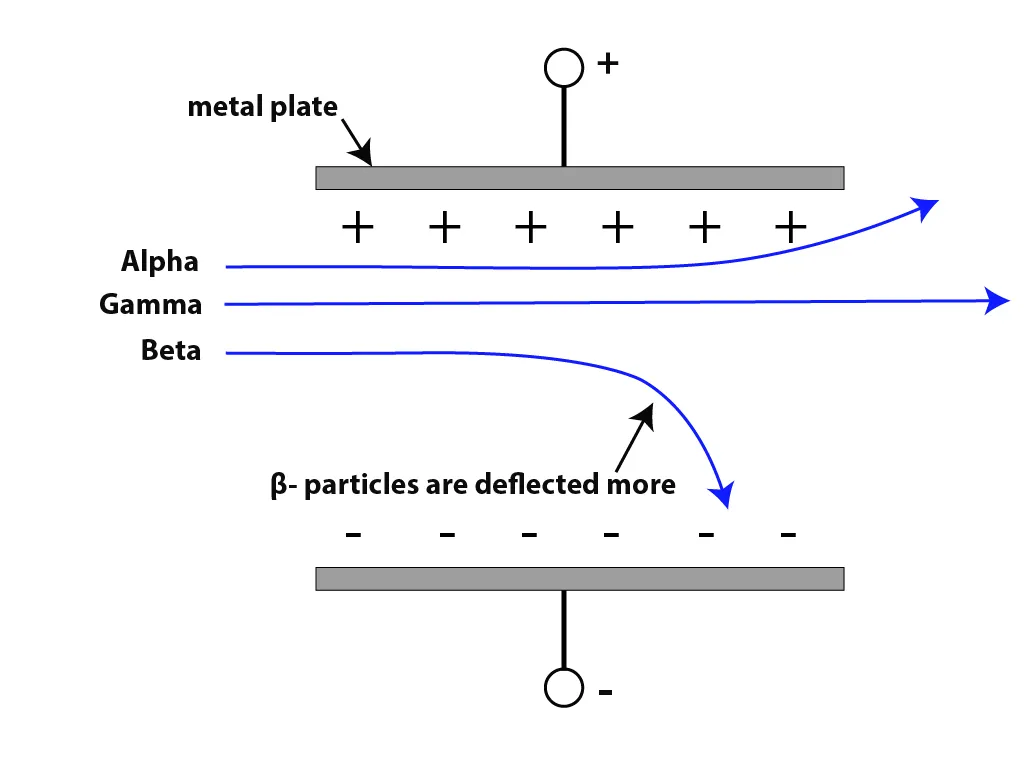
Figure 2: Deflection of α-particles and β⁻-particles and γ-rays in an electric field
b. Magnetic field.
- α-particles and β⁻-particles are charged, so when they move, they produce an electric current.
- This means they experience a force when moving through a magnetic field – this is known as the motor effect.
- The direction of the force (i.e., the direction of deflection) can be predicted using Fleming's Left-Hand Rule.
- For α-particles (positive charge), the current direction is the same as the particle's motion.
- For β⁻-particles (negative charge), the current direction is opposite to the particle's motion.
- Because of their opposite charges, α and β particles are deflected in opposite directions in a magnetic field.
- γ-rays, being uncharged, are not deflected in either electric or magnetic fields.
Fleming's Left-Hand Rule
To apply this rule:
- Thumb → Direction of Force (i.e., deflection)
- First Finger → Direction of Magnetic Field (from North to South)
- Second Finger → Direction of Current (direction of positive charge flow)
| Radiation | Charge | Deflection in Electric/Magnetic Field |
|---|---|---|
| α | +2 | Small deflection (heavier, slower) |
| β⁻ | –1 | Large deflection (lighter, faster) |
| γ | 0 | No deflection |
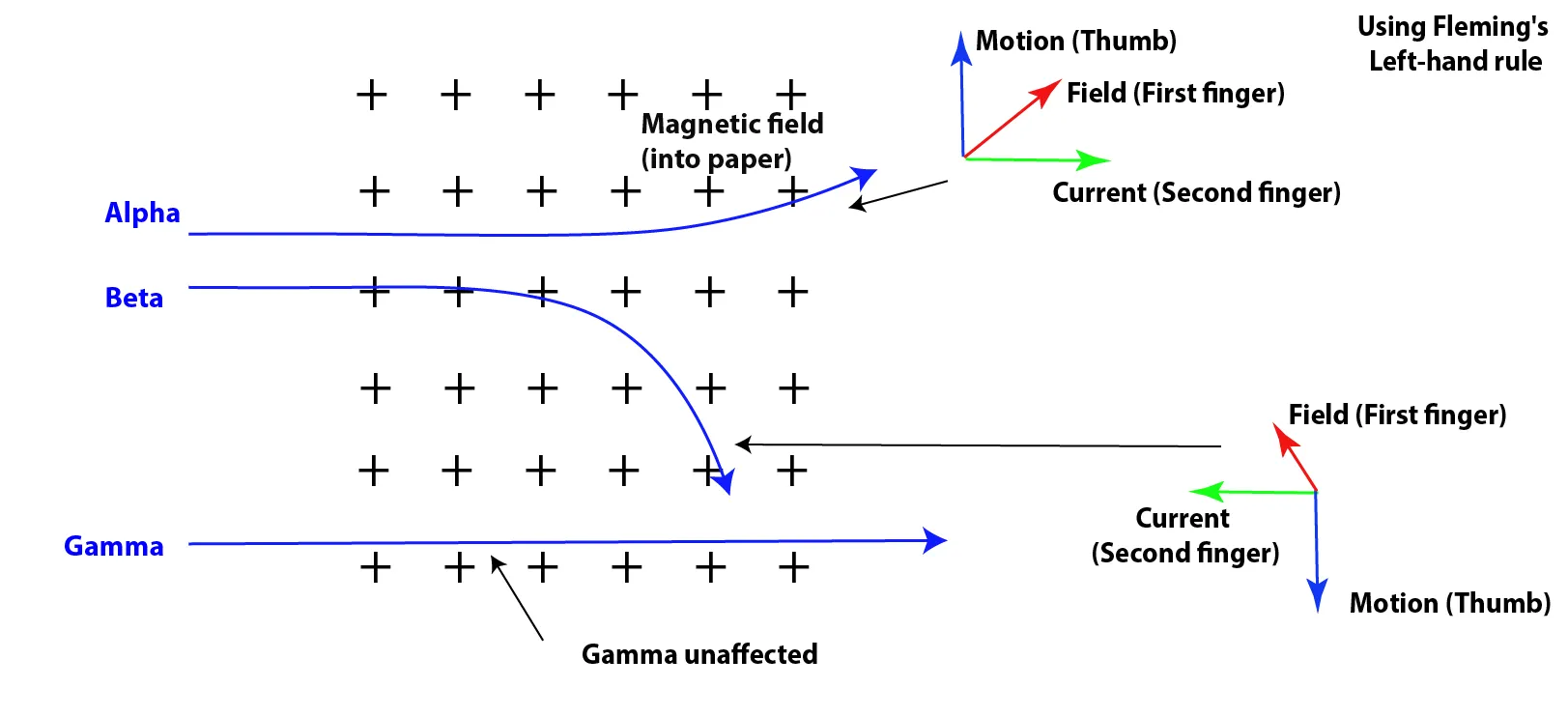
Figure 3: Deflection of α-particles and β⁻-particles and γ-rays in a magnetic field
4. Explaining Relative Ionising Effects Using Kinetic Energy and Charge
- The ionising ability depends on two main factors:
- Kinetic energy of the particle
- Charge of the particle
| Particle | Charge | Mass | Kinetic Energy | Ionising Ability |
|---|---|---|---|---|
| α | +2 | 4 × proton mass | Low | Very strong |
| β⁻ | –1 | 1/1840 × proton mass | Higher | Moderate |
| γ | 0 | 0 (no mass) | Very high | Very weak |
- What is the Ionising Effect?
The ionising effect refers to the ability of radiation to remove electrons from atoms, creating ions. This process is called ionisation. Ionising radiation can damage living tissues, which is why it is important in health and safety contexts.
Ionising Effects and Penetration of α, β, and γ Radiation
| Type of Radiation | Ionising Effect | Penetration Power | Reason |
|---|---|---|---|
| Alpha (α) | Very strong | Low (stopped by paper or skin) | Large mass and +2 charge → frequent collisions with matter → easily knocks out electrons |
| Beta (β⁻) | Moderate | Medium (few mm of aluminium) | Smaller mass and –1 charge → fewer collisions, but still enough energy to ionise |
| Gamma (γ) | Weak | High (several cm of lead) | No charge (EM wave) → little interaction with matter, travels far without ionising much |
Explanation Summary
- Alpha particles
→ Heaviest and most charged → interact strongly with matter
→ Knock electrons off atoms easily
→ High ionising power, short range because they lose energy quickly in collisions - Beta particles
→ Lighter and negatively charged
→ Still knock off electrons, but cause less ionisation
→ Fewer collisions than alpha → greater range, up to a few metres in air - Gamma rays
→ Electromagnetic waves with no charge or mass
→ Pass through matter with very little interaction
→ Can still ionise atoms, but least ionising
→ Travel far before losing energy
Summary Table: Properties of α, β, and γ Radiation
| Emission | Nature | Charge | Penetration | Ionising Effect | Deflection |
|---|---|---|---|---|---|
| α | Helium nucleus (2p, 2n) | +2 | Stopped by paper or a few cm of air | Very strong | Slight |
| β⁻ | High-speed electron | –1 | Stopped by few mm of aluminium | Moderate | Strong |
| γ | Electromagnetic wave (γ photon) | 0 | Stopped by thick lead/concrete | Very weak | None |
