5.1 The nuclear model of the atom
5.1.1 The atom
Core Content
1. Structure of an Atom
- An atom consists of:
- A positively charged nucleus (containing protons and neutrons)
- Negatively charged electrons orbiting the nucleus in energy levels (shells)
- The nucleus is tiny compared to the atom but contains most of its mass
- The atom is mostly empty space between the nucleus and electrons
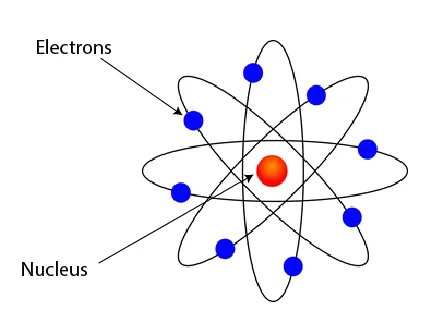
Figure 1: The nuclear model of the atom
2. Formation of Ions
- Positive ions form when atoms lose electrons
Na → Na⁺ + e⁻
Shows a sodium atom losing one electron to become a positively charged sodium ion.
- Negative ions form when atoms gain electrons
Cl + e⁻ → Cl⁻
Chlorine atom gaining one electron to become a negatively charged chloride ion.
- Ions are charged because the number of protons ≠ electrons
Supplement Content
3. Alpha Particle Scattering Experiment (Rutherford's Gold Foil Experiment)
- Procedure:
- Alpha particles (positively charged) were fired at a thin gold foil
- A detector measured the deflection angles of the particles
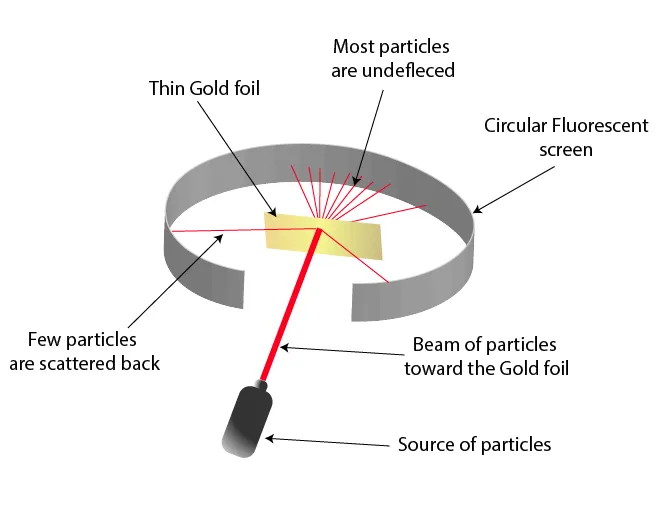
Figure 2: Experimental setup of alpha particles striking gold foil, showing deflections
- Observations:
- Most alpha particles passed straight through (Figure 3)
- A few were slightly deflected
- Very few (1 in 8000) bounced back
- Conclusions (Evidence for the Nuclear Model):
- (a) Mostly empty space: Most alpha particles passed through because atoms are not densely packed (unlike the plum pudding model)
- (b) Concentrated mass: The few deflections showed a tiny, dense nucleus containing most of the atom's mass
- (c) Positive nucleus: Rebounded alpha particles indicated a positively charged nucleus repelling them
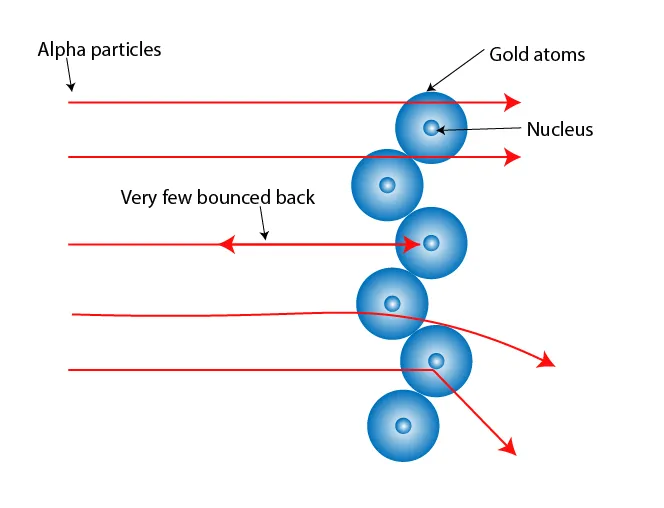
Figure 3: Alpha particles passing through empty space, with a few deflected near the nucleus
Key Notes
- Scale of the Atom:
- Atom diameter: ~10⁻¹⁰ m
- Nucleus diameter: ~10⁻¹⁵ m (100,000 times smaller)
IGCSE Physics Syllabus Reference: 5.1 The nuclear model of the atom
