5.1 The nuclear model of the atom
5.1.2 The nucleus
Core Content
1. Composition of the Nucleus
- Nucleus contains:
- Protons (+1 charge)
- Neutrons (0 charge)
- Negatively charged electrons orbit the positively charged nucleus (negligible mass)
2. Relative Charges & Masses
| Particle | Position | Relative Charge | Relative Mass | Charge (C) | Mass (kg) |
|---|---|---|---|---|---|
| Proton | In nucleus | +1 | 1 | +1.6×10-19 | 1.67×10-27 |
| Neutron | In nucleus | 0 | 1 | 0 | 1.67×10-27 |
| Electron | Orbiting nucleus | -1 | ~0 (1/1836) | -1.6×10-19 | 9.11×10-31 |
3. Proton Number (Z) & Nucleon Number (A)
- Proton number (Z): Number of protons (defines the element)
- Nucleon number (A): Protons + neutrons
- Neutron number (N): N = A - Z
4. Nuclide Notation
A
Z
X
Example: 146C (Carbon)
- Proton number = 6
- Nucleon number = 14
- Neutron number = 8 (14 - 6 = 8)
- Electrons = 6 (if neutral)
5. Isotopes
- Atoms of the same element (same Z) with different neutron numbers (N)
- Example:
- Hydrogen isotopes: 11H (0 neutrons), 21H (1 neutron), 31H (2 neutrons)
- Helium isotopes: 42He (2 neutrons), 32He (1 neutron)
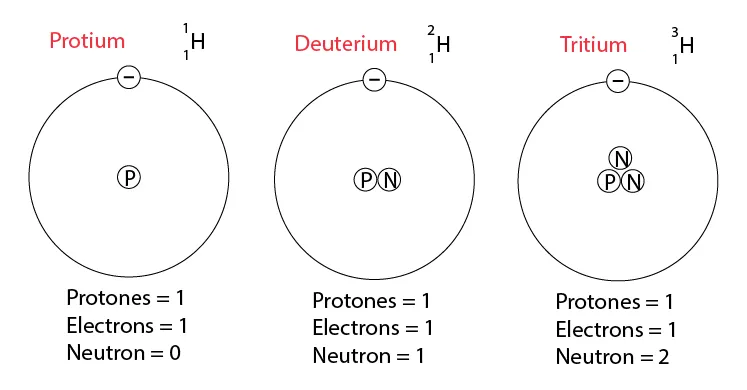
Figure 4: Isotopes of hydrogen showing protium, deuterium, and tritium
To determine if an element is an isotope, compare its atomic structure to the standard form of the element:
- Same number of protons (same element)
- Different number of neutrons (different mass number)
Supplement Content
6. Nuclear Fission & Fusion
a. Fission: Splitting heavy nuclei (e.g., uranium-235)
23592U + 10n → 9236Ba + 14156Kr + 310n + energy
- Both proton and nucleon numbers balance:
- Proton number: 92 + 0 = 36 + 56 + 3(0) → 92 = 92 ✓
- Nucleon number: 235 + 1 = 92 + 141 + 3(1) → 236 = 236 ✓
- Releases energy via chain reactions (used in nuclear power)

Figure 5: Uranium-235 fission reaction producing barium, krypton, and neutrons
b. Fusion: Joining light nuclei (e.g., in stars)
21H + 21H → 32He + 10n + energy
- Both proton and nucleon numbers balance:
- Proton number: 1 + 1 = 2 + 0 → 2 = 2 ✓
- Nucleon number: 2 + 2 = 3 + 1 → 4 = 4 ✓
- Generates more energy than fission by joining nuclei at very high temperatures
- How stars like the Sun produce energy
7. Relationship Between Proton Number and Nuclear Charge/Mass
- Nuclear charge = Proton number (Z) × (+1)
- Charge: The "charge" of a nucleus (the center of an atom) comes from its protons. Each proton has a +1 charge. So, if a nucleus has one proton, its charge is +1. All forms of hydrogen have one proton, so they all have a +1 charge.
- Nuclear mass ≈ Nucleon number (A) (protons + neutrons)
- Protium (11H):(a type of hydrogen) has 1 proton and 0 neutrons, so its mass is 1.
- Deuterium (21H): (another type of hydrogen) has 1 proton and 1 neutron, so its mass is 2.
- Tritium (31H): (yet another type of hydrogen) has 1 proton and 2 neutrons, so its mass is 3.
- Essentially, different versions of hydrogen have the same charge because they have the same number of protons, but they have different masses because they have different numbers of neutrons.
8. Mass-Energy Equivalence (E=mc²)
E = mc²
- Energy released (E) = mass lost (m) × speed of light squared (c²):
- The total mass of the particles before a fusion or fission reaction is found to be slightly more than the total mass after the reaction. The mass which is lost is converted to energy, and Einstein’s equation allows us to calculate the amount of energy released:
- The speed of light (speed of light(c) = 3×108 m/s) squared is a huge number, so even a very small loss of mass will produce a large amount of energy.
- Explains energy production in both fission and fusion
IGCSE Physics Syllabus Reference: 5.1.2 The nucleus
