2.3 Transfer of thermal energy
2.3.1 Conduction
Experiments to Demonstrate Good and Bad Thermal Conductors:
- Good Conductors: Metals like copper and aluminum transfer heat quickly.
- Example: A metal rod heated at one end will transfer heat to the other end quickly, making the whole rod hot.
- Bad Conductors (Insulators): Materials like wood, plastic, and air do not transfer heat well.
- Example: Place a metal spoon and a wooden spoon in a cup of hot water. The metal spoon becomes hot faster, while the wooden spoon remains cool.
Experiment 01:
To demonstrate the properties of good and bad thermal conductors.
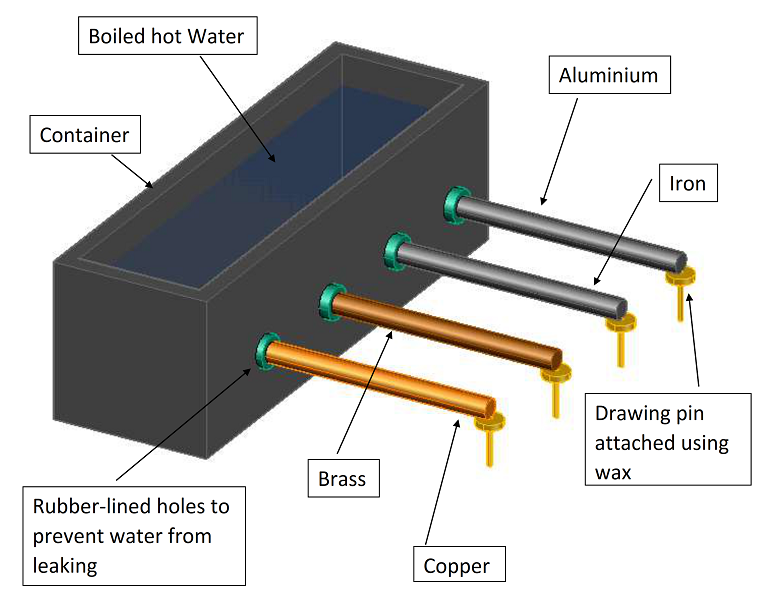
Step 01 : Take 4 different types of metal rods with same length and cross sectional area.
Step 02 : Prepare the experiment as above. Using a container with four rubber-lined holes in the side.
Step 03 : Insert the rods through the holes, pushing them in the same amount.
Step 03 : attach a drawing pin to the end of each using the same mass of wax.
Step 04 : Fill the container with boiling water and start the stopwatch.
Step 05 : Measure the time taken for each of the drawing pin to fall off from their respective rod and compare the times.
The rod that allowed the pin to fall off fastest is the best conductor.
Supplement
Thermal Conduction in Solids:
- Lattice Vibrations: In solids, atoms are arranged in a lattice. When heated, atoms vibrate more vigorously and pass on their energy to neighboring atoms.
- Free Electrons in Metals: In metals, free (delocalized) electrons move through the lattice and carry thermal energy quickly, making metals good conductors.
Why Gases and Liquids are Poor Conductors:
- In gases and most liquids, particles are far apart compared to solids, so they do not collide often. This makes conduction less efficient.
Intermediate Conductors:
- Some materials, such as ceramics or glass, conduct thermal energy better than insulators like wool but not as well as good conductors like metals.
2.3.2 Convection
Convection in Liquids and Gases:
- Convection is a major method of thermal energy transfer in liquids and gases (fluids). It occurs when warmer, less dense fluid rises and cooler, denser fluid sinks, forming a cycle.
Explaining Convection in Terms of Density Changes:
- When fluid is heated, it expands and becomes less dense, causing it to rise. As it cools, it contracts, becomes denser, and sinks. This sets up a convection current.
Experiment 02:
Air Convection Currents Experiment
Step 01 : Set up the box and chimneys as shown in the picture. Light a candle and place it under one of the glass chimneys.
Step 02 : The air around the candle’s flame will be warmer than the rest of the air.
Step 03 : Light the touch paper and hold it above the other chimney.
Observation : You’ll notice that instead of the smoke rising, which is what it usually does, it gets sucked down through the chimney and emerges from the other chimney.
This is because of convection. The air heated by the candle becomes less dense, and rises.
The colder air moves to replace it, causing the smoke to be sucked along with it.

2.3.3 Radiation
Thermal Radiation is Infrared Radiation:
- Thermal energy is transferred by infrared radiation, which all objects emit. Hotter objects emit more infrared radiation than cooler objects.
Radiation Does Not Require a Medium:
- Unlike conduction and convection, radiation does not require a medium (air, liquid, etc.) to transfer energy. For example, the heat from the Sun reaches Earth through the vacuum of space by radiation.
Effect of Surface Color and Texture on Infrared Radiation:
- Black surfaces: Absorb and emit infrared radiation more efficiently than white surfaces.
- Shiny surfaces: Reflect infrared radiation, making them poor emitters and absorbers.
- Dull surfaces: Are better absorbers and emitters compared to shiny ones.

Experiment 03:
To show the properties of good and bad emitters of infra-red radiation.
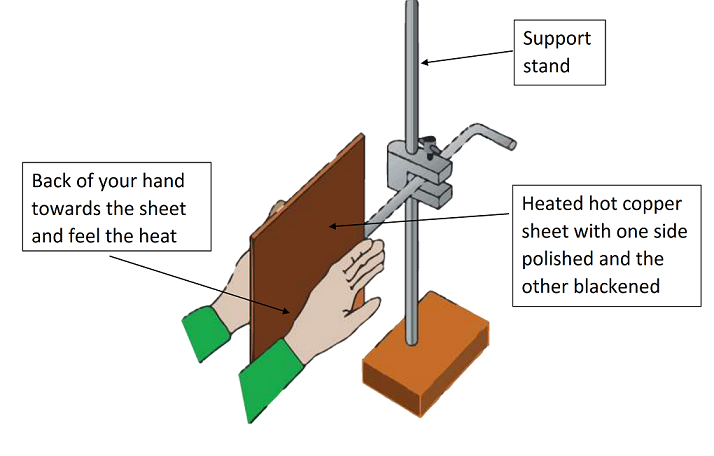
Step 01 : Prepare the equipment as show above. (The copper sheet has previously been heated strongly with a Bunsen burner)
Step 02 : Keep you hand close the sheet as shown above (do not touch)with same distance
Observation : The hand next to the back surface feels much hotter than the hand next to the polished surface.
Conclusion : black surfaces emit thermal energy radiation more than polished surfaces.
Experiment 04:
To show the properties of good and bad absorbers of infra-red radiation.
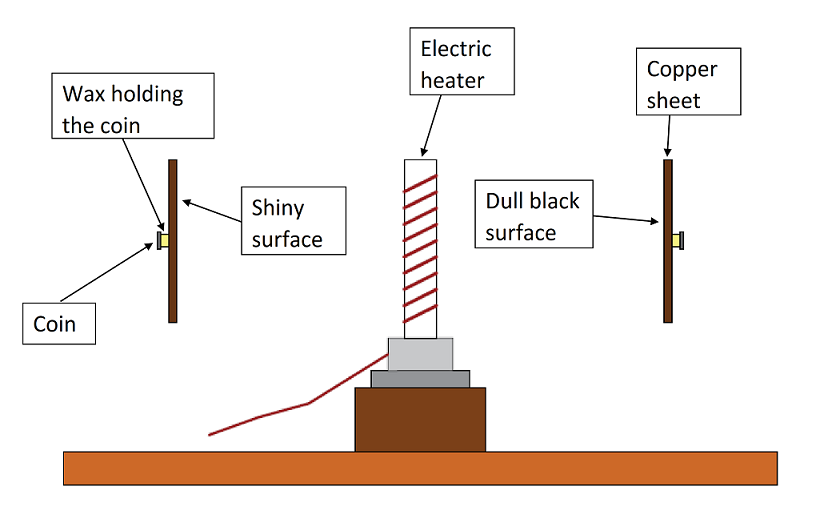
Step 01 : Prepare the equipment as shown above
Step 02 : Place two copper sheets same distance from the electric heater as shown above so each receive same amount of heat radiation
Step 03 : Turn on the heater and start the stop watch at the same time.
Observation : After few min the wax on the black sheet melts and the coin falls off. The shiny surface reflects a lot of the radiation, so it takes way more time to melt the wax.
Supplement
Constant Temperature and Energy Transfer:
- For an object to remain at a constant temperature, the rate at which it loses energy must equal the rate at which it gains energy.
Energy Imbalance:
- If an object receives more energy than it transfers away, its temperature increases. If it transfers energy away faster than it receives energy, its temperature decreases.
Factors Controlling Earth’s Temperature:
- The Earth’s temperature depends on the balance between incoming solar radiation (from the Sun) and outgoing infrared radiation (emitted by Earth).
- Factors such as greenhouse gases trap more heat, causing the Earth’s temperature to rise. Deforestation, cloud cover, and ice reflectivity also play roles in controlling this balance.
2.3.4 Consequences of Thermal Energy Transfer
Basic Everyday Applications of Conduction, Convection, and Radiation:
(a) Heating Objects (e.g., Kitchen Pans):
- Conduction: Metal pans conduct heat from the stove to the food because metals are good conductors of heat. The handle is often made from insulating materials (like plastic) to prevent burns.
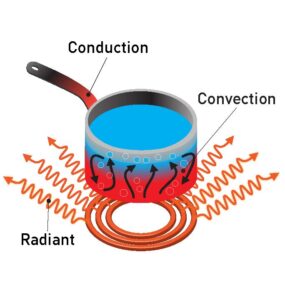
(b) Heating a Room by Convection:
- Convection: In a heated room, warm air rises and cooler air sinks, creating convection currents. Heaters warm the air, which rises and circulates the room, distributing heat.

Complex Applications and Consequences of Conduction, Convection, and Radiation:
(a) A Fire Burning Wood or Coal:
- Conduction: The heat is transferred through the solid wood or coal by conduction as it burns.
- Convection: Hot gases and air rise above the fire, creating convection currents that distribute the heat.
- Radiation: Infrared radiation from the flames and hot coals directly heats objects around the fire.
(b) A Radiator in a Car:
- Conduction: The radiator conducts heat from the hot engine coolant to its metal surface.
- Convection: Air flows over the radiator surface, and the heated air rises away, cooling the radiator and allowing cooler air to continue the process.
- Radiation: The radiator also radiates heat, transferring some thermal energy away from its surface without direct contact.

Sir when u will update waves I really need notes for waves