2.2 Thermal properties and temperature
2.2.1 Thermal expansion of solids, liquids and gases
Thermal Expansion of Solids, Liquids, and Gases (Qualitative)
Thermal Expansion: When materials are heated, they expand. This happens because particles move faster and spread out.
- Solids: Particles are tightly packed and vibrate more as temperature increases, causing slight expansion.
- Liquids: Particles are less tightly packed than solids, so they expand more than solids when heated.
- Gases: Particles are far apart and move freely, so gases expand the most when heated, as particles move faster and occupy more space.
Applications and Consequences of Thermal Expansion
Applications:
- Thermometers: Use the expansion of liquids (like mercury or alcohol) to measure temperature.

- Bridges and Railways: Expansion joints are used to allow for thermal expansion without causing damage.


- Bimetallic Strips: Used in thermostats to bend when heated and regulate temperature.
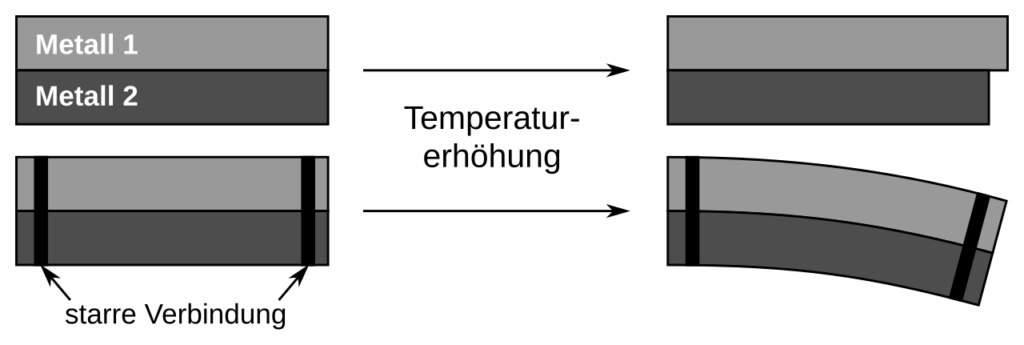
Consequences:
- Cracking of materials: Expansion and contraction of materials (like roads or building structures) can cause cracks.
- Power lines: Sag in hot weather due to expansion.

Relative Magnitudes of Expansion: Solids, Liquids, and Gases
- Solids: Particles are closely packed in a fixed structure, so expansion is small because particles only vibrate more within their positions.
- Liquids: Particles are less tightly packed, allowing them to move around more freely, leading to moderate expansion.
- Gases: Particles are far apart and move very freely, so gases expand the most as they have much more space to spread out when heated.
Particle Explanation:
- In solids, particles vibrate more but cannot move much, so expansion is small.
- In liquids, particles can move past each other, leading to greater expansion.
- In gases, particles are free to move in all directions, leading to the largest expansion.
2.2.2 Specific heat capacity
Rise in Temperature and Increase in Internal Energy
- Internal Energy: The total energy stored in an object due to the motion and arrangement of its particles.
- Effect of Temperature Rise: When the temperature of an object increases, its internal energy increases because the particles move faster.
- Solids: Particles vibrate more rapidly.
- Liquids and Gases: Particles move faster and more freely.
Increase in Temperature and Kinetic Energy of Particles
- Kinetic Energy of Particles: The energy particles have due to their motion.
- As Temperature Rises:
- The average kinetic energy of all particles in the object increases.
- In Solids: Particles vibrate more intensely within fixed positions.
- In Liquids: Particles move more rapidly while sliding past one another.
- In Gases: Particles move faster and spread further apart.
- Temperature and Kinetic Energy: The temperature of an object is directly related to the average kinetic energy of its particles. A higher temperature means higher average kinetic energy.
Thermal Capacity (Heat Capacity)
The thermal capacity of an object is the amount of heat energy required to raise the temperature of that object by 1 °c.
The greater the thermal capacity of an object, the more heat energy it takes to raise its temperature.
The thermal capacity is also equal to the amount of heat energy an object will give out when it cools by 1 °c.
Thermal Capacity = mc
‘m’ is the mass, (Kg)
‘c’ is the specific heat capacity unit is joules per kilogram per degree Celsius (J/kg °C)
Change of Energy (ΔE)=mcΔT
‘T’ is the Temperature change
‘c’ is the specific heat capacity unit is joules per kilogram per degree Celsius (J/kg °C)
$$c={{ΔE}\over{mΔT}}$$
‘T’ is the Temperature change
‘ΔE’ = Change of Energy
‘c’ is the specific heat capacity unit is joules per kilogram per degree Celsius (J/kg °C)
Define specific heat capacity:
Specific heat capacity is the energy required per unit mass per unit temperature
increase.
Measure the specific heat capacity of a substance (Experiment)
Determine the specific heat capacity of vegetable oil
Step 01 : Place a beaker on a balance and press zero
Step 02 : Add the oil to the beaker and record the mass of the oil (For example let say the mass of the oil is 900g)
Step 03 : Then place a thermometer and an immersion heater into the oil.
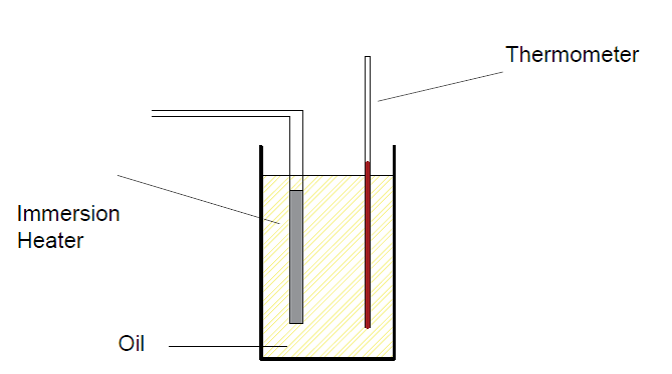
Step 04 : Read the starting temperature using the thermometer
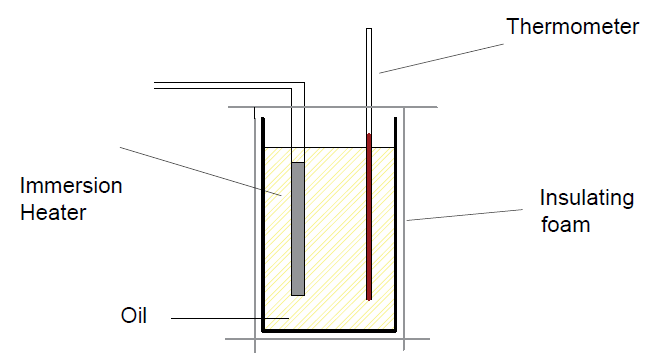
Step 05 : Wrap the beaker in insulating foam to reduce thermal energy transfer to the surrounding
Step 06 : Connect a Joulemeter and a power pack to the immersion heater as show below.
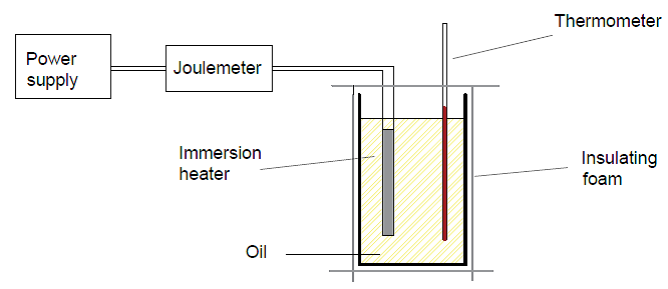
Step 07 : Leave the setup for 30 minutes
Step 08 : Read the number of joules of energy that passed into the immersion heater.
Step 09 : Read the final temperature of Oil and hence calculate the change in temperature.
Step 10 : Now use the equation to calculate Specific heat capacity
$$c={{ΔE}\over{mΔT}}$$
2.2.3 Melting, boiling and evaporation
Melting and Boiling (Core)
- Melting: The process where a solid turns into a liquid.
- Energy is absorbed by the particles, which increases their kinetic energy, causing them to break free from their fixed positions.
- Important: During melting, temperature does not change until all the solid has melted, even though energy is still being absorbed.
- Boiling: The process where a liquid turns into a gas throughout the entire volume of the liquid.
- Particles absorb energy, increasing their kinetic energy until they can overcome the attractive forces and escape as gas.
- Important: Boiling happens at a specific temperature, and like melting, the temperature does not change during the process until all the liquid has boiled.
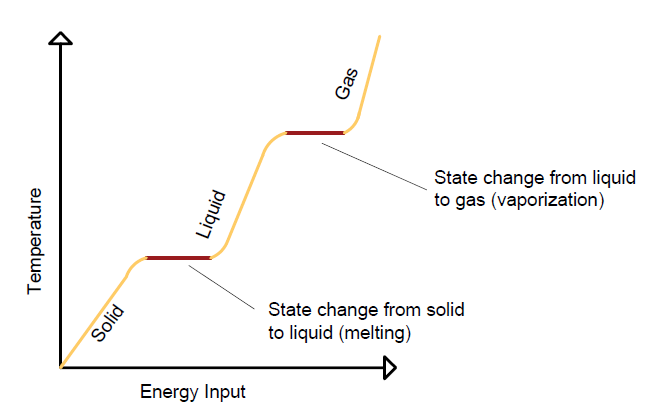
Melting and Boiling Temperatures for Water
- Melting point of water: 0°C (273 K) at standard atmospheric pressure.
- Boiling point of water: 100°C (373 K) at standard atmospheric pressure.
Condensation and Solidification.
- Condensation: Gas to liquid.
- As gas cools, particles lose energy and move closer together, forming a liquid. It’s the reverse of boiling.
- Solidification: Liquid to solid.
- When a liquid cools, its particles lose kinetic energy and settle into a fixed position, forming a solid. This is the reverse of melting.
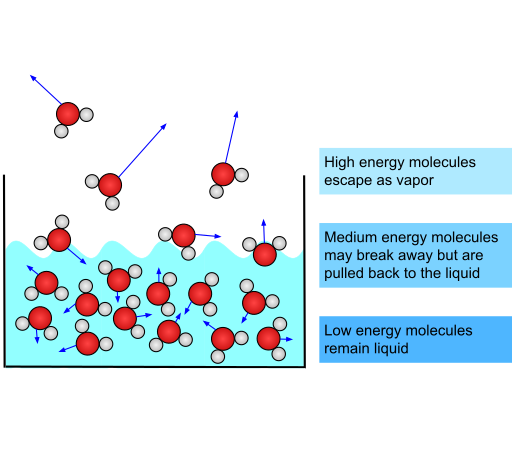
Evaporation.
- Evaporation: The process where particles at the surface of a liquid escape into the gas phase.
- Particles with higher kinetic energy escape first, leaving behind particles with lower energy.
- Key point: Unlike boiling, evaporation happens at any temperature.
Evaporation Causes Cooling
- Evaporation cools the liquid because the most energetic particles leave, reducing the average energy of the remaining particles.
- The decrease in energy leads to a lower temperature.
Differences Between Boiling and Evaporation
| Boiling | Evaporation |
|---|---|
| Occurs at a specific temperature (boiling point) | Occurs at any temperature |
| Happens throughout the liquid | Happens only at the surface |
| Requires external heat input | Does not require external heat input |
| Rapid process | Slower process |
Factors Affecting Evaporation
- Temperature: Higher temperature increases particle energy, speeding up evaporation.
- Surface Area: Larger surface area allows more particles to escape, increasing the rate of evaporation.
- Air Movement: Moving air (wind) carries away evaporated particles, preventing saturation and promoting further evaporation.
Cooling of an Object in Contact with an Evaporating Liquid
- When an object is in contact with a liquid that is evaporating, the liquid takes energy from the object to fuel evaporation. This energy loss causes the object to cool down.
- Example: Sweating cools the body because the evaporation of sweat removes heat from the skin.

CINEMA in book it was 800 pgs